 Symbol P
Symbol P
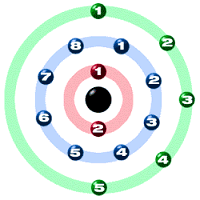
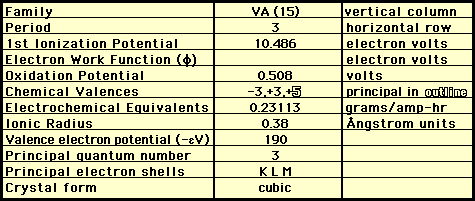
Atomic Number 15
Atomic Mass 30.97376
Electron Configuration 2-8-5
W h a t ' s i n
a N a m e ?
Phosphorus got its name because it glows in the dark. "Phosphorus" is from the Greek words phôs (light) and phoros (bearer), meaning "bringer of light," and the ancient name for the planet Venus when it appears before sunrise. Its symbol is P, from phosphorus.
H i s t o r y
Phosphorus was first isolated in 1669 by German physician Hennig Brand (ca. 1630-1692). Brand was convinced that the key to changing metals into gold could be found in urine. While heating and purifying urine, Brand discovered phosphorus! It was the first time anyone had discovered an element unknown to ancient peoples.
Before Brand's discovery, phosphorus and its various compounds have been mentioned in old manuscripts as phosphorescents, or materials that glow in the dark.
G e n e r a l P r o
p e r t i e s
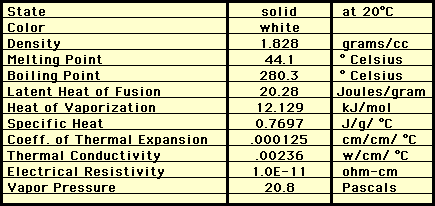
Phosphorus is a nonmetal. As a gas it is colorless, but as a solid it can be a silvery white or a red depending on the how is bonded with itself. It is a vital part of living thing, like in human nervous tissue. It burns when exsposed to air.


Phosphorus exists in at least 3 allotropic forms. The three main allotropes are named for their colors: white (or yellow) phosphorus, red phosphorus, and black phosphorus. The most common and reactive of which is white (or yellow) phosphorus which looks like a waxy, transparent solid with a garlic-like odor. It is very reactive and will spontaneously inflame in air so it is stored under water. Sometimes it appears yellow because it contains traces of red phosphorus!
Red phosphorus is much less reactive and is one of the components on the striking surface of a match book. It is a red powder and does not dissolve in most liquids. Red phosphorus is formed by exposing white phosphorus to sunlight or heating it under pressure to above 275oC.
Black phosphorus is also produced by heating white phosphorus in the presence of a mercury catalyst and a seed crystal of black phosphorus. Black phosphorus is the least reactive, does not ignite easily and has the least commercial value.
W h e r e i s
P h o s p h o r u s F o u n d a n d U s e d ?
Commercially, phosphorus compounds are used in the manufacture of phosphoric acid (H3PO4) (found in soft drinks and used in fertilizer compounding). Other compounds find applications in fireworks and, of course, phosphorescent compounds which glow in the dark.




Scientists use phosphorus to make baking soda. Go take a look in your refrigerator. Maybe your mom keeps baking soda in there to take away the smell of the food. When you see that white powder you'll know that scientists used phosphorus to make it.
Phosphorus is also used to make dishes. Fine china is very expensive because a lot of special procedures go into making it. Phosphorus is one of the special elements that are used to make that fine china.
You can find lots of phosphorus in fireworks. When phosphorus gets hot it burns really brightly. The bright sparks and flashes are usually because of that phosphorus.
Phosphorus is a very important element in fertilizers. Plants need small amounts of phosphorus to grow up healthy. People also need phosphorus and they get it by eating plants.
Scientists use phosphorus when they make glass. If you look at your computer or television they have glass monitors. So much is made of glass. It's everywhere you look. A lot of that glass was made with help from phosphorus.
| 
