|
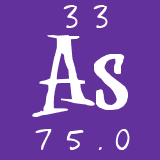
Symbol
As
Atomic
Number 33
Atomic
Mass 74.9216
Electron
Configuration [Ar] 3d104s24p3
Density @ 293 K
5.72 g/cm3
Melting
Point 1090 K
Boiling
Point 886 K (sublimes)
1st
Ionization Energy 946.5 kJ/mole
Structure
Rhombohedral
Atomic
radius 120 pm
H i s t o r y
Arsenic compounds have
been known since the days of Ancient Greece and Rome when arsenic sulfide
(As2S3) was used by physicians and poisoners. Since arsenic can be produced
from its ores very easily, many early craftspeople may have seen the element
without realizing what it was. It was first recognized as an element by
alchemists. Although it is not certain where and when arsenic was actually
discovered, credit for the discovery usually goes to alchemist Albert the Great
(Albertus Magnus, 1193-1280). In 1250 A.D. he heated a common compound of
arsenic, As2S3, with soap and formed nearly pure arsenic. It wasn’t until the
mid-seventeenth century that arsenic became well known as an element.
S o u r c e s

Arsenic is occasionally
found as a free element, but it is usually found in a number of minerals, the
most common one being mispickel. The abundance of arsenic in the Earth’s crust
is thought to be about 5 parts per million. Arsenic can be found in France,
Germany, Italy, Romania, Siberia, China, Chile, Mexico, Belgium, Namibia, and
the Philippines. The United States does not produce any arsenic.
G e n e r
a l P r o p e r t i e s

~ Elemental arsenic occurs in two
solid modifications: yellow and gray or metallic. It is a steel gray, very
brittle, crystalline, metalloid.
~ Arsenic does not melt when heated.
Instead, it changes directly into a vapor (sublimation). However, under high
pressure, arsenic can be forced to melt at 814 C.
~ Arsenic is stable in dry air, but
the surface oxidizes slowly in moist air to give a bronze tarnish and finally a
black covering to the element.
~ Arsenic does not react with water in
the absence of air under normal conditions.
~ When heated in air, arsenic combines
with oxygen to form arsenic oxide (As2O3). A blue flame is produced and the
compound gives off a distinctive garlic-like odor.
B i o l o
g i c a l R o l e
Arsenic may be a necessary
ultratrace element for humans. It is a necessary ultratrace element for red
algae, chickens, rats, goats, and pigs. A deficiency of arsenic results in
inhibited growth.
U s e s

The most infamous use of
arsenic is as a poison. However, arsenic can now be detected during autopsy, so
this use of the element has become a legend of the past. These days the most
important use of arsenic is in the preservation of wood. It is used in the form
of a compound called chromated copper arsenate (CCA) and is added to wood used
to build houses and other wooden structures. CCA prevents organisms from
growing in the wood and causing it to rot. Arsenic is also used as a weed
killer and rat poison. Arsenic has been used to improve the roundness of lead
shot. Trace amounts of arsenic are alloyed with lead in storage batteries.
Arsenic is also used in the manufacture of high-efficiency solar cells. Alloys
of gallium, arsenic, and phosphorous are used in the semiconductor industry for
the production of light-emitting diodes (LEDs) in watches, clocks, calculators,
and numerous other instrument displays.
H a z a r
d s & R i s k s
Arsenic compounds are very
poisonous to plants and animals. In low doses, arsenic causes nausea, vomiting,
and diarrhea. In larger doses, it results in abnormal heartbeat, damage to
blood vessels, and a feeling of “pins and needles” in hands and feet. Small
corns or warts may begin to develop on the palms of the hands and the soles of
the feet. Direct contact with the skin can cause redness and swelling. Long
term exposure can cause cancer. Inhalation results in lung cancer. If arsenic
is swallowed, cancer may develop in the bladder, kidneys, liver, and lungs. In
large doses, arsenic can be deadly. Despite the danger of arsenic, it is found
in pesticides, wood preservatives, and many household products.
I n t e r
e s t i n g F a c t s
~
In its pure form, arsenic costs $320 per 100g.
~ Arsenic compounds were mined by the
early Chinese, Greek, and Egyptian civilizations. No doubt they discovered its
toxic properties early on.
~ Arsenic’s name appears to derive
from the Latin word arsenicum and the Greek word arsenikos,
meaning “masculine” or “male.” In earlier times it was believed that metals had
different sexes.
~ In 1989, the total usage of arsenic
in the world was 52,380 tons of As2O3, with approximately 28,530 tons imported
by the United States. Pesticides and wood preservatives accounted for over 80
percent of this use.
~ Arsenic makes up 50 ppb by weight
and 4 ppb by atoms in a human being.
~ During the Middle Ages, arsenic
compounds were often used to commit murder. At the time, it was difficult to
detect the presence of arsenic in the body, so the person who was murdered was
thought to have died of pneumonia
|