 Symbol Bi
Symbol Bi
Atomic Number 83
Atomic Mass 208.98038
Electron Configuration 2-8-18-32-18-5
W h a t ' s i n
a N a m e ?
From the German word wissmuth which means "white mass." Its symbol is Bi from bismuth.
H i s t o r y
Bismuth was recognized as a metal by early observers, including George Agricola in the 16th century but bismuth was confused with tin and lead. It was not until 1753 that Claude Geoffroy the Younger showed it to be distinct from lead.
G e n e r a l P r o p e r t i e s
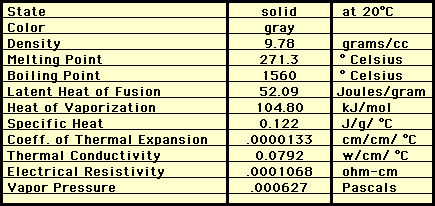
Bismuth is the heaviest non-radioactive naturally occurring element. It is a hard, brittle metal with an unusually low melting point (271oC).
Bismuth is a silver-white crystalline, brittle metal with a pinkish tinge.
Physical Properties
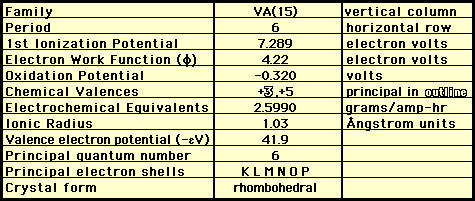
Chemical Properties
W h e r e i s
B i s m u t h F o u n d a n d U s e d ?
Bismuth is a relatively rare metal found in the earth's crust at about the same abundance as silver and almost never occurring in the native state. It is usually associated with copper, lead, tin, wolfram, silver, and gold ores.
Major producers of Bismuth are Peru, Japan, Mexico, Bolivia and Canada. Bismuth
is found free in nature and in such ores as bismuth glance and bismite which are primarily found in South America but are rare in the United States.
In the United States bismuth is obtained as a by-product in refining lead,
copper, tin, silver, and gold ores.
 Its unusual property to expand upon solidification makes it useful in type-metal alloys and for castings. The most important use of bismuth is in the manufacture of low-melting alloys which is used in electrical fuses and in automatic fire
alarm and sprinkler systems. For these uses bismuth is mixed with lead, tin or iron to form fusible metals, which melt at low temperatures. Its unusual property to expand upon solidification makes it useful in type-metal alloys and for castings. The most important use of bismuth is in the manufacture of low-melting alloys which is used in electrical fuses and in automatic fire
alarm and sprinkler systems. For these uses bismuth is mixed with lead, tin or iron to form fusible metals, which melt at low temperatures. For example, in fire alarms plugs made of these alloys will melt from the heat of the fire which then turns on the systems.
Bismuth is also used in foundries and in nuclear reactors. Its alloys give sharp impressions when they are used to make objects by casting in molds. Bismuth has no biological role. However, it has been used as a medicine (tripotassium dicitratobismuthate) for treatment of stomach upsets. When combined with antibiotics it is used for treatment of some stomach ulcers.
It is also found in hemorrhoid creams such as Anusol cream and Hemocaneas as bismuth oxide.
 
  Bismuth Subsalicylate is useful in treating gastric disorders such as colitis, diarrhea, and peptic ulcers. Pepto BismolŪ gets its name from bismuth.
Bismuth Subsalicylate is useful in treating gastric disorders such as colitis, diarrhea, and peptic ulcers. Pepto BismolŪ gets its name from bismuth.
Bismuth is used in a wide variety of pigments ranging from cosmetics to the coloration of glass, decorative glazing, enameling and "metallic" automotive paints, as well as UV or heat-absorbing coatings for outer-space and strategic applications. Bi-citrate is used in
hair coloring dyes to improve color and to deodorize the stabilizing additives.
H a z a r d s a n d
R i s k s
Some medical experts warn against using certain substances containing bismuth because these substances have been
found to cause toxic reactions in people which can cause mild kidney damage.
| 
