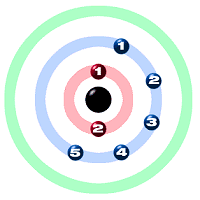
 Symbol N Symbol N
Atomic Number 7
Atomic Mass 14.0067
Electron Configuration 2-5
W h a t ' s i n
a N a m e ?
Nitrogen gets its name from a mineral known as "niter" (potassium nitrate), from
which it can be prepared.
H i s t o r y
Until the late 1700s gases were poorly understood by chemists. They
often wondered what air was made of. In the 1770's, a Scottish physician
and chemist Daniel Rutherford (1749-1819) performed a simple experiment with
which he discovered nitrogen. Rutherford began with an empty bottle that
he turned upside down in a pan of water, so that the air was trapped. A
burning candle was placed inside the bottle with the trapped air, causing the
water to rise a bit. Why did this happen? The part of the air that
seemed to "disappear" when the candle was burned was oxygen gas, and the part of
the air that did not "disappear" Rutherford discovered was nitrogen.
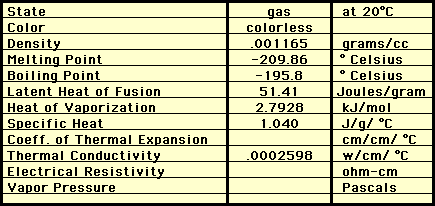
PHYSICAL PROPERTIES
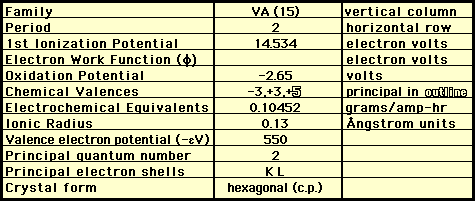
CHEMICAL PROPERTIES
WHERE IS NITROGEN USED AND FOUND?
Nitrogen is a fairly common element in the Earth's crust.
It is by far the most important element of the Earth's atmosphere, making up
about 78.084 percent. The greatest use of nitrogen is in ammonia which is
used for fertilizer production and to produce nitric acid. Nitrogen
gas is used where an inert atmosphere (one without oxygen) is needed, such as in
an ordinary light bulb. Nitrogen is also used to protect historic
documents such as the Declaration of Independence. Liquid nitrogen is
used by the oil industry to build up pressure in wells to force crude oil
upward. It is also possible to buy large containers of liquid nitrogen
which can be used to freeze materials like food. This is how most of the
frozen foods in a grocery store are used. Nitrogen is also used in
explosives.
HEALTH EFFECTS
Nitrogen is absolutely essential to all living organisms.
It is an important part of all protein molecules which, among other functions,
are the building material in all kinds of cells Nitrogen is also used to
make up nucleic acids which store an organism's genetic information. Nitrogen
gas is not toxic, but, in principle, pure nitrogen could asphyxiate the body by
denying it access to oxygen.
INTERESTING FACTS
~ Nitrogen gas makes up about 78% of the atmosphere on
Earth but only 3% of the atmosphere on Mars.
~ In the Oklahoma City bombing, an ammonium nitrate bomb
destroyed the Murrah Building. The explosion resulted from a mixture of
4,800 pounds of ammonium nitrate and fuel oil from twenty plastic drums.
~ Nitrogen oxides (from combustion) are one cause of toxic
rain.
| 
