|
|
|
|
|
... ... ... ... ... ...
|
THE BASICS
HISTORY: Carbon was once known as diamond or graphite. Diamonds have been known as early as 1200 B.C. according to ancient Hindu writings. The name diamond is derived from a corruption of the Greek word, adamas meaning "the invincible". The first recognition of graphite is obscure. It was confused with other minerals of similar appearance, like moydenite (MoS2). One name for graphite was plumbago, like lead. In 1779, Scheele demonstrated that graphite oxidized into carbon dioxide. The name graphite, which comes from the Greek verb graphain, "to write", originated with Werner in 1789. Not until modern times was the identity and role of carbon completely understood. SOURCE: Carbon is found in abundance in the sun,
stars, comets and atmospheres of most planets. Graphite is found naturally in many locations. Diamond is found in the form of microscopic crystals in some meteorites. Natural diamonds are found in the mineral, kimberlite, in South Africa, Arkansas and elsewhere. Diamonds are now also being recovered from the ocean floor off the Cape of Good Hope.
About 30% of all industrial diamonds used in the
United States are made
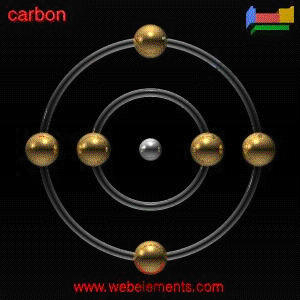
synthetically. OCCURRENCE: Carbon ranks nineteenth in the order of abundance of the elements comprising about 0.2% of the earth's outer crust. In the earth's atmosphere, carbon is present as carbon dioxide. Though it is widely distributed in nature mainly in the combined form, only minor amounts are found in the free or elemental state. It is present as main constituent of all animal and vegetable matter. Coal, petroleum, and natural gas are also composed essentially of carbon. Various minerals, such as limestone, dolomite, and marble, as well as certain marine deposits such as oyster shells, all contain carbon in the form of carbonates. Carbon plays a vital role in the carbon life cycle. Carbon dioxide from the air, together with water, is absorbed by plants and converted into carbohydrates in the process of photosynthesis. Animals consume the carbohydrates, returning the carbon dioxide to the atmosphere by the processes of respiration, excretion, fermentation and decay under bacterial action. USES: Carbon is unique among the elements in the vast number and variety of compounds it can form. With hydrogen, oxygen, nitrogen, and other elements, it forms very large numbers of compounds. Carbon atoms also combine with other carbon atoms. This ability to form chains is unique to carbon, and is thought to be an important reason for the dependence of life on this element. It is also an indispensable source of such varied everyday products as nylon and petrol, perfume and plastics, shoe polish, DDT and TNT Carbon is a large element in industry. By far, the greatest single use of carbon is in the form of coke for the iron and steel industry. The major portion of this coke is used in the reduction of iron ore in blast furnaces. In the rubber industry, the major applications for carbon blacks are in the printing ink, paint, pencils, paper, and plastic industries. Minor amounts are used in the manufacture of dry cells, carbon brushes, and insulation. The largest single application for gas phase activated carbons is in the recovery of volatile organic solvents from air or vapor mixtures. Another large application is in the purification and separation of natural and industrial gases. Main applications for manufactured graphite are found as components for rockets, missiles, and other aerospace vehicles. BIOLOGICAL INFO: Carbon is the basis of all life as part of the DNA molecule. There are more than a million known carbon compounds, many thousands of which are vital to organic and life processes. No toxic effects appear to be associated with carbon in its elemental form. On the other hand, many of the more common carbon compounds exhibit strong toxicological effects. Principal among these are carbon monoxide, carbon dioxide, hydrogen cyanide, alkali cyanide, carbon tetrachloride, and carbon disulfide. Carbon monoxide, an odorless gas, is extremely toxic, behaving as an asphyxiate. Compared to oxygen, it is not only more readily absorbed, but also more firmly bound by the hemoglobin of the blood. The capacity of the blood to carry oxygen to the vital parts of the body is thereby reduced, leading to brain damage, heart disease and pneumonia. Carbon dioxide is less toxic and behaves chiefly as a simple asphyxiate and narcotic. Hydrogen cyanide and the alkali cyanides are extremely toxic, functioning as protoplasmic poisons by inhibiting tissue oxidation. Acute exposure to the vapors of carbon tetrachloride can result in damage to both the liver and kidneys. Carbon disulfide is a powerful narcotic, but its chronic effects are more serious. Excessive exposure can lead to permanent damage to the nervous system. GENERAL INFO: Carbon is found free in nature
in three allotropic forms; amorphous, graphite and diamond. A fourth form
known as "white" carbon is now thought to exist. Graphite is one
of the softest known materials, and diamond is one of the hardest. This
difference is purely because of the arrangement of atoms in each of the
two forms. In graphite, hexagonal rings are joined together to form
sheets, and the sheets lie one on top of the other. In diamond, the atoms
are arranged tetrahedrally in a vast continuous array. White carbon is
a transparent birefringent material produced during the sublimation of
graphite at low pressures.
|