Sodium is perhaps the most characteristic alkali metal, reacting violently with water and rapidly with the oxygen in air.
Most People have never seen sodium metal, but it is It is the sixth most abundant element in the earth's crust. Sodium combines with other elements to form compounds used in everyday life
-
Table Salt (NaCl)
-
Baking Soda
-
Baking powder
-
Soaps and Detergents
-
Aspirin
BASIC FACTS
Date of discovery: 1807
Discoverer: Sir Humphry Davy- an English Chemist who found a way to extract sodium from its compounds.
Name origin: Soda (Na2CO3)
Symbol origin: From the Latin word Natrium
Uses: medicine, agriculture
Obtained From: table salts and other foods
PHYSICAL PROPERTIES
Sodium in a silvery white metal with a waxy appearance that is so soft it can easily be cut with a knife.
Boiling point: 881.4˚C
The Electron Configuration for sodium is
1s2 2s2p6 3s1
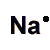
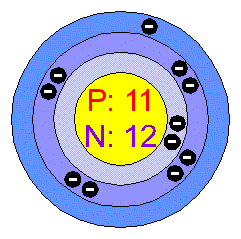
Atomic Structure