-BASICS FACTS-
Common Compounds:
●Potassium chloride- essential for plant growth
●Potassium hydroxide- assists in making ceramics, detergents, glass, soaps, and textile dyes; occurs when reacting with water
●Potassium nitrate- black gunpowder and matches
●Potassium bromides and Iodides- used in medicine
Physical Properties:
- melting point- 760 °C
- boiling point- 63.7 °C
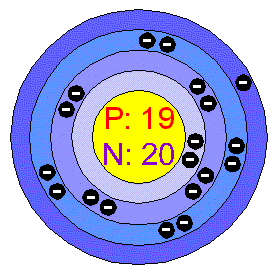
>Atomic Structure of Potassium
Fun and Interesting Facts:
- Potassium is never found as a pure metal
-Potassium salt is found in large deposits in Carlsbad, New Mexico Saskatchewan, Canada, and Stassfurt, East Germany
- leading producers of Potassium salts are: Canada, East Germany, France, Russia, the United States, and West Germany
-0.07% of the ocean is make up of Potassium Chloride
-elemental Potassium is stored in a petroleum liquid to keep out moisture and oxygen in the air
Atomic Number: 19
Atomic Weight: 39.102
Electron Configuration:
1s2 2s2 2p6 3s2 3p6 4s1
- Potassium is found in: fertilizer, fruits and vegetables (especially in bananas)
>One of the main vitamins in Bananas is Potassium
-a Potassium deficiency in the human body is characterized by muscle weakness, fatigue, mental confusion, irritability, weakness, heart disturbances, and problems in nerve conduction and muscle contraction. This is caused by a low diet of fresh fruits and vegetables, while still high in Sodium.
-Potassium is extremely important in electrolyte function since 95% of your body's cells are made up of Potassium