Physical Properties:
Rubidium is a very soft
silvery-white metal that violently combines with water, setting fire to the
liberated hydrogen, and may ignite in air. When it burns, its flame is a
yellowish-violet color. It is solid at 298K and liquid at 313K. It forms
amalgams with mercury and alloys with gold, cesium, potassium and sodium.
History:
Rubidium was discovered by Robert Bunsen and Gustave Kirchoff in 1861 in Germany. Its name is from the Latin word Rubidious meaning red, the color that its salt burns
Source
Rubidium is the 16th most abundant element in the
earth's crust. It occurs
in the minerals pollucite, carnallite, leucite and lepidolite, from which it
is recovered commercially. Potassium minerals and brines also contain this
element and are a further commercial source.
Uses
Rubidium is used little outside research. It is easily ionized so was
considered for use in ion
engines, but was found to be less effective than
cesium. It has been proposed for use as a working fluid for vapor turbines
and in thermoelectric generators. It is used as a
photocell
component and in
special glasses. Because rubidium can be easily ionized, it has been
considered for use in "ion engines" for space vehicles; however, cesium is
somewhat more efficient for this purpose. It is also proposed for use as a
working fluid for vapor turbines and for use in a thermoelectric generator
using the magnetohydrodynamic principle where rubidium ions are formed by
heat at high temperature and passed through a
magnetic field. These conduct
electricity and act like an amateur of a generator thereby generating an
electric current. Rubidium is used as a getter in vacuum tubes and as a
photocell component. RbAg4I5
is important, as it has the highest room conductivity of any known ionic
crystal. At 20oC
its conductivity is about the same as dilute sulfuric acid. This suggests
use in thin film
batteries and other applications. The picture below show
the use of laser cooled rubidium atoms in an atomic clock, which will
increase the clocks accuracy by a factor of 30.

Rubidium has five principle energy levels. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1. This configuration leaves it with a single valence electron in its outer shell. This electron is easily lost to form a more stable existence.
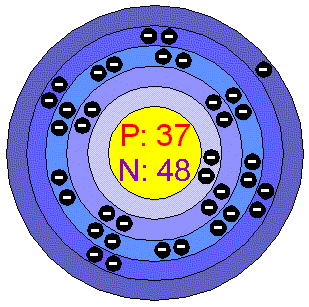
In 1861, rubidium was discovered spectroscopically when a very distinctive, ruby-red colour spectral line was observed. The new element was given the name rubidium, from the Latin name rubidus, meaning "deep red".

This is the physical appearance Rubidium. As you can see it is very soft and a silvery color.