Symbol: Be
Atomic Mass: 9.012
Atomic Number: 4
# of Protons:4
# of Electrons:4
# of Neutrons:5
Melting Point: 1560 K (1287°C or 2349°F)
Boiling Point: 2744 K (2471°C or 4480°F)
Electronegativity:
1.57 (Pauling); 1.47 (Allrod
Rochow)
Density: 1.85 grams per cubic
centimeter
The name beryllium comes from the
Greek beryllos,
beryl, from
Prakrit veruliya,
from
Pāli veuriya;
possibly from or simply akin to a
Dravidian source
represented by
Tamil veiruor, viar,
"to whiten, become pale." [1]
At one time beryllium was referred to as glucinium
(from
Greek glykys,
sweet), due to the sweet taste of its
salts. This element
was discovered by
Louis Vauquelin in
1798 as the oxide
in
beryl and in
emeralds.
Friedrich Wöhler
and
A. A. Bussy
independently isolated the metal in
1828 by reacting
potassium and
beryllium chloride.
Dangers of Beryllium
Beryllium and its salts are
toxic
substances and potentially
carcinogenic.
Chronic
berylliosis
is a
pulmonary
and
systemic
granulomatous
disease caused by exposure to
beryllium. Acute beryllium disease
in the form of
chemical
pneumonitis was first
reported in Europe in 1933 and in
the United States in 1943. Cases of
chronic berylliosis were first
described in 1946 among workers in
plants manufacturing
fluorescent
lamps in
Massachusetts.
Chronic berylliosis resembles
sarcoidosis
in many respects, and the
differential diagnosis is often
difficult. Although the use of
beryllium compounds in fluorescent
lighting tubes was discontinued in
1949, potential for exposure to
beryllium exists in the nuclear and
aerospace industries and in the
refining of beryllium metal and
melting of beryllium-containing
alloys, the manufacturing of
electronic devices, and the handling
of other beryllium-containing
material.
Early researchers tasted
beryllium and its various compounds
for sweetness in order to verify its
presence. Modern diagnostic
equipment no longer necessitates
this highly risky procedure and no
attempt should be made to ingest
this substance. Beryllium and its
compounds should be handled with
great care and special precautions
must be taken when carrying out any
activity which could result in the
release of beryllium dust (lung
cancer is a possible
result of prolonged exposure to
beryllium laden dust).
This substance can be handled
safely if certain procedures are
followed. No attempt should be made
to work with beryllium before
familiarization with correct
handling procedures.
A successful test for beryllium
on different surface areas has been
recently developed. The procedure
uses fluorescence when beryllium is
bound to sulfonated
hydroxybenzoquinoline to detect up
to 10 times lower than the
recommended limit for beryllium
concentration in the work place.
Fluorescence increases with
increasing beryllium concentration.
The new procedure has been
successfully tested on a variety of
surfaces.
Discovery of Beryllium
Discovery of the element in the mineral beryl, was reported by
Nicolas Louis Vauquelin in 1789.
Commercial value of Beryllium
1969 Bertrandite mine established in the United
States providing a significant raw materials source
1977 Effects of inflation rates, increased energy
costs, and additional costs associated with complying with air
emission
standards results in increased prices
1979 Beryllium metal price set by one producer
1988 Purchase of beryllium metal for the National
Defense Stockpile (NDS)
1990 Conversion of NDS beryl ore to beryllium metal
for the NDS
1991 Recession, dissolution of the Soviet Union
Beryllium found in nature
Pure beryllium is not found
in nature. Beryllium compounds can be found in mineral
rocks, soil, coal, oil, and volcanic dust.
(ball of beryllium below)
 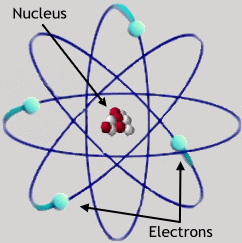 (diagram
of a beryllium atom) (diagram
of a beryllium atom)
|
Properties of Beryllium
Beryllium is a toxic bivalent element, steel gray,
strong, light-weight, primarily used as hardening agent
in alloys. Beryllium has one of the highest melting
points of the light metals. It has excellent thermal
conductivity, is nonmagnetic, it resists attack by
concentrated nitric acid and at standard temperature and
pressures beryllium resist oxidation when exposts to
air.
Beryllium is used as an alloying agent in the
production of beryllium-copper. Thanks to their
electrical and thermal conductivity, high strenght and
hardness, non magnetic properties, good resistance,
dimensional stability over a wide temperature range
beryllium-copper alloys are used in many applications. A
typical application of beryllium-copper alloys is in the
defense and aerospace industries.
Beryllium is also used in the field of X-ray detection
diagnostic (it is transparent to X-rays) and in the
making of various computer equipment. |
|
|
|

|
Here is a brief
description of beryllium.
Beryllium is a Group 2 (IIA) element. At ordinary
temperatures, beryllium resists oxidation in air. Its
ability to scratch glass is probably due to the formation of
a thin layer of the oxide. Aquamarine and emerald are
precious forms of the mineral beryl, [Be3Al2(SiO3)6].
Uses of Beryllium
- Beryllium is used as an
alloying
agent in the production of beryllium-copper
because of its ability to absorb large
amounts of heat. Beryllium-copper alloys
are used in a wide variety of
applications because of their
electrical
and
thermal
conductivity, high strength
and
hardness,
nonmagnetic properties, along with good
corrosion and fatigue resistance. These
applications include the making of spot-welding
electrodes,
springs,
non-sparking tools and
electrical
contacts.
- Due to their stiffness, light
weight, and dimensional stability over a
wide temperature range, beryllium-copper
alloys are also used in the defense and
aerospace industries as light-weight
structural materials in high-speed
aircraft, missiles, space vehicles and
communication
satellites.
- Thin sheets of beryllium foil are
used with
X-ray
detection diagnostics to filter out
visible light and allow only X-rays to
be detected.
- Beryllium is an effective p-type
dopant in III-V compound semiconductors.
It is widely used in materials such as
GaAs, AlGaAs, InGaAs, and InAlAs grown
by molecular beam epitaxy (MBE).
- In the field of X-ray lithography
beryllium is used for the reproduction
of microscopic
integrated
circuits.
- In the
telecommunications
industry, Beryllium is made into tools
that are used to tune the highly
magnetic
klystrons
used for high power
microwave
transmissions for safety.
- Because it has a low
thermal neutron
absorption cross section, the nuclear
power industry uses this metal in
nuclear reactors
as a neutron reflector and moderator.
- Beryllium is used in nuclear weapons
for similar reasons. For example, the
critical mass
of a plutonium sphere is significantly
reduced if the plutonium is surrounded
by a beryllium shell.
- Beryllium is sometimes used in
neutron sources,
in which the beryllium is mixed with an
alpha emitter such as 210Po,
226Ra,
239Pu
or 241Am.
- Beryllium is also used in the making
of
gyroscopes,
various
computer
equipment, watch springs and instruments
where light-weight, rigidity and
dimensional stability are needed.
-
Beryllium oxide
is useful for many applications that
require an excellent heat conductor,
with high strength and hardness, with a
very high melting point, and that acts
as an electrical insulator.
- Beryllium compounds were once used
in
fluorescent
lighting tubes, but this use
was discontinued because of
berylliosis
in the workers manufacturing the tubes
- The
James Webb Space
Telescope[3]
will have 18 hexagonal beryllium
sections for its mirrors. Because JWST
will face a temperature of −240 degrees
Celsius (30 kelvins), the mirror is made
of beryllium, a material capable of
handling extreme cold better than glass.
Beryllium contracts and deforms less
than glass — and thus remains more
uniform — in such temperatures.
- Beryllium is also used in the
Joint European
Torus fusion research
facility, to condition the plasma facing
components.[4]
- Beryllium has also been used in
tweeter
construction by the company
Focal-JMlab
on its flagship Utopia Be series as an
alternative to
titanium
and
aluminium,
largely due to its lower density and
greater rigidity.[5]
|
|


 (diagram
of a beryllium atom)
(diagram
of a beryllium atom)