
![]()
Name: Zinc
Symbol: Zn
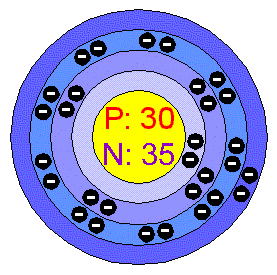
Atomic Number: 30
Atomic Mass: 65.39 amu
Melting Point: 419.58 °C (692.73 °K, 787.24396 °F)
Boiling Point: 907.0 °C (1180.15 °K, 1664.6 °F)
Number of Protons/Electrons: 30
Number of Neutrons: 35
Classification: Transition Metal
Crystal Structure: Hexagonal
Density @ 293 K: 7.133 g/cm3
Color: bluish
Number of Energy Levels:
4
First Energy Level: 2
Second Energy Level: 8
Third Energy Level: 18
Fourth Energy Level: 2
Facts
Date of Discovery: 1746
Discoverer: Andreas Marggraf
Name Origin: From the German word zin (meaning tin)
Uses: metal coating, rust protection, brass, bronze, nickel
Obtained From: zinc blende, calamine
![]()
·
History: The U.S. penny is not made entirely of
copper. In fact, 98% of the penny is Zinc with a copper coating!!! That’s
common cents!
·
The U.S. consumes more than 1 million
metric tons of zinc annually.
·
The Average person will use 730 pounds of
zinc in their life according to the U.S. Bureau of Mines. That’s
a ton of Zinc!!!
·
Zinc is primarily used as a coating on
iron and steel to protect against rust and corrosion. Architects love Zinc for
its low maintenance and high durability.
· Physics: Zoom!!! Zoom!!! Zoom!!! Zinc makes cars last longer. It is used to make die cast parts such as door handles, and locks. There is even a half pound of zinc in each tire to cure the rubber.
·
The Energizer Bunny! Zinc stores six
times as much energy per pound as other batteries. The zinc air battery can
power a car up to 120 mph, and can run a laptop computer for 12 hours on one
charge. (That’s 10 times better than the old laptop batteries).
·
Zinc is even used in toys. For example toy
factories use over 40,000 tons of zinc
annually on things like toy vehicles.
