
RADON

![]()
The element was discovered in 1900 by Dorn, who called it radium emanation. In 1908 Ramsay and Gray, who named it niton, isolated the element and determined its density, finding it to be the heaviest known gas. It is essentially inert and occupies the last place in the zero group of gases in the Periodic Table. Since 1923, it has been called radon.
Twenty isotopes are known. Radon-22, from radium, has a half-life of 3.823 days and is an alpha emitter; Radon-220, emanating naturally from thorium and called thoron, has a half-life of 55.6 s and is also an alpha emitter. Radon-219 emanates from actinium and is called actinon. It has a half-life of 3.96 s and is also an alpha emitter. It is estimated that every square mile of soil to a depth of 6 inches contains about 1 g of radium, which releases radon in tiny amounts into the atmosphere. Radon is present in some spring waters, such as those at Hot Springs, Arkansas.
On the average, one part of radon is present ot 1 x 1021 part of air. It is formed from the natural radioactive decay of uranium in rock, soil, and water. At ordinary temperatures radon is a colorless gas; when cooled below the freezing point, radon gives off a brilliant phosphorescence which becomes yellow as the temperature is lowered and orange-red at the temperature of liquid air. It has been reported that fluorine reacts with radon, forming a fluoride. Radon clathrates have also been reported.
Radon is still produced for therapeutic use by a few hospitals by pumping it from a radium source and sealing it in minute tubes, called seeds or needles, for application to patient. This has been stopped as hospitals can get the seeds directly from suppliers, who make up the seeds with the desired activity for the day of use. Also used in earthquake prediction and the treatment of cancer.
Care must be taken in handling radon, as it is a radioactive material. The main hazard is from inhalation of the element and its solid daughters which are collected on dust in the air. Good ventilation should be provided where radium, thorium, or actinium is stored to prevent build-up of the element. Radon build-up is a health consideration in uranium mines. Recently radon build-up in homes has been a concern. Many deaths from lung cancer are caused by radon exposure. Its is the second leading cause of lung cancer in the United States.
Vital Statistics
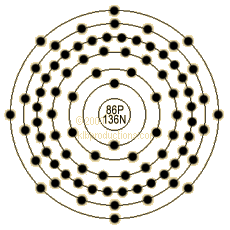
| Atomic Number | 86 |
| Atomic Mass | 222.0 amu |
| Melting Point | 202.15 °K |
| Boiling Point | 211.35 °K |
| Number of Neutrons | 136 |
| Density @STP | 9.73 g/cm3 |
| Color | colorless |
| Electron Configuration | [Xe]6s24f145d106p6 |
 |