






Description:
Neon was discovered by the British chemists Sir William Ramsay and Morris M. Travers shortly after their discovery of the element krypton in 1898. Its name is derived from the Greek word neos or "new". Like krypton, neon was discovered through the study of liquefied air. It is the fourth most abundant element in the universe and even though only 0.0018% of the earth's atmosphere is made up of neon, it is still fifth most abundant in this atmosphere. Neon is highly inert and forms no known compounds, although there is some evidence that it could form a compound with fluorine. No stable compounds of neon are known to date. Natural neon is a mixture of three isotopes. Six other unstable isotopes are known. Neon also forms an unstable hydrate. In a vacuum discharge tube, neon glows reddish orange. Of all the rare gases, the discharge of neon is the most intense at ordinary voltages and currents.
______________________________
Properties:
Atomic Symbol: Ne
Atomic Number: 10
Atomic Weight: 20.1797
Group Number: 8
Standard State: gas at 298K
Color: colorless
structure of neon:
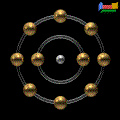
Electron Shell Configuration: 1s2 2s2 2p6
Thermal Data:
Melting Point: -248.67 °C
Boiling Point: -246.048 °C
Specific Heat: 0.904 J/g K
Heat of Fusion: 0.3317 kJ/mol
Heat of Vaporization: 1.7326
Atomic Radius: 0.51 Ă
Crystal structure: Cubic: face centered
______________________________
example of neon lights:

Uses:
Neon is used in making the common neon lights, which account for its largest use. A neon light consists of a glass tube filled with neon or some other inert gas. An electric current is passed through the tube causing neon atoms to break apart. After a fraction of a second, the parts recombine. When this happens, they give off neon light. Neon lighting was invented by French chemist Georges Claude (1870-1960). Claude displayed his first neon sign at the Paris Exposition of 1910. He sold his first neon sign to a Paris barber two years later. By the 1920s, neon lighting had become popular in many parts of the world and continues to be today. It is also used to make high-voltage indicators, lightning arrestors, wave meter tubes, and TV tubes. Neon tubes are also used to detect electric currents and are used in the manufacture of lasers which have many uses in industry and medicene. Liquid neon has been used in the cryogenic refrigerant, because it has over 40 times the refrigerant power than liquid helium and three times the refrigerant than hydrogen. It is compact, inert, and is less expensive than helium when it meets refrigeration requirements costing about $2.00/Liter.