Francium
In the
United States
it was at one time called virginium!!!!
Date of Discovery:
1939
Discoverer: Marguerite Derey
Name Origin: After France
Uses: No uses known
Obtained From: decay of actinium
Marguerite Perey of the Curie Institute in Paris reported in 1939 the discovery of a new radioactive element. She found it while analyzing the radioactive decay products of actinium. She named the new element francium after her country.
Interesting Facts
- Francium does not have any stable isotopes.
- There is at most one ounce of francium in the whole earth at any given time as a result of the decay of other radioactive elements.
- It is the most unstable of the first 103 elements in the periodic table. Its longest lived isotope has a half life of 22 minutes.
- Francium is the heaviest simple atom

This sample of uraninite contains some francium because of a steady-state decay chain. An estimate suggests there is about 10-20 grammes of francium (about 1 atom!) at any one time.
Electron Configuration
1s2 2s2p6 3s2p6d10 4s2p6d10f14 5s2p6d10 6s2p6 7s1
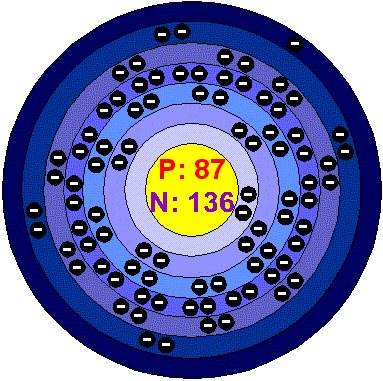 Atomic Structure!!!
Atomic Structure!!!
- Because it is so rare, its chemical and physical properties are not known, but it is believed to resemble cesium
- Francium is extremely rare; its most stable isotope (half-life about 22 minutes) occurs naturally, to a very limited extent, in uranium minerals. More than 30 other isotopes of francium are known.