![]()


Named for the Scottish village of Strontian
|
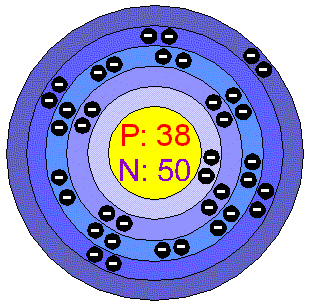
Atomic #- 38 Atomic Weight- 87.62 Electron Configuration - 1s22s22p63s23p64s23d104p65s2
Intensive Physical Properties - |
 |
||||||||||||||||||||||||||||||||||||||||||||
| Being an Alkaline Earth Metal, Strontium forms a strong base when placed in water (An Alkaline solution) | |||||||||||||||||||||||||||||||||||||||||||||
|
Physical Properties Strontium is a lightweight, silvery-white metal. It is less dense than Calcium and is not found free in nature. Strontium is silver-white when freshly cut but rapidly turns a yellowish color through oxidation. |
 |
||||||||||||||||||||||||||||||||||||||||||||
|
Common Chemical Reactions Strontium, like Magnesium, forms and oxide layer (giving Strontium its yellowish color) . Once ignited, Strontium burns with a bright white flame and form an oxide (SrO) and a nitride (Sr3O2) The surface layer of Sr reacts with Oxygen and Nitrogen in the air to form an oxide that protects it from corrosion 2Sr(s) + O2(g)
3Sr(s) + N2(g)
When placed in water, Strontium sinks to the bottom and then begins to react with the water. Small bubbles of H2 appear on the surface. Sr reacts quicker than Ca but slower than Ba Sr(s)
+ 2H2O(g)
Strontium is very reactive towards the halogens such as chlorine, Cl2 or bromine, Br2, and Iodine, I2. and burns (reacts) to form the dihalides strontium(II) chloride, MgCl2 and strontium(II) bromide, MgBr2, respectively. These reactions occur around 400 Co Sr(s)
+ Cl2(g)
Sr(s)
+ Br2(g)
Sr(s)
+ I2(g)
|
|||||||||||||||||||||||||||||||||||||||||||||
|
A Few Isotopes
|
Strontium's radioisotopes have varying length
half lives. Ranging from 20 min. to 30 yr. Ex: 89Sr has a half life of 52.50 days Strontium90 has a half life of 29 years and is a part of nuclear fallout. |
||||||||||||||||||||||||||||||||||||||||||||
|
Sources of Strontium Strontium is never found as a free element in nature. Like other Alkaline metals, it readily combines with other elements Strontium can be found in minerals such as Celestite and Strontianite |
|||||||||||||||||||||||||||||||||||||||||||||
|
Uses of Strontium
|
 |
||||||||||||||||||||||||||||||||||||||||||||
|
Strontium in Biology Strontium has no positive role in biology. However, because it chemically resembles Calcium it can be found bones where Calcium should be. The same occurs with 90Sr which was a radioactive product of nuclear testing during the 1950s |
Excess= bone degradation Deficiency= no side effects |
||||||||||||||||||||||||||||||||||||||||||||
|
History of Strontium Adair Crawford in 1790 recognized a new mineral (strontianite) in samples of witherite (a mineral consisting of barium carbonate, BaCO3) from Scotland. It was some time before it was recognized that strontianite contained a new element. Strontianite is now known to consists of strontium carbonate, SrCO3. The element itself was not isolated for a number of years after this when strontium metal was isolated by Davy by electrolysis of a mixture containing strontium chloride and mercuric oxide in 1808.
|
Discovered by: Adair
Crawford |
||||||||||||||||||||||||||||||||||||||||||||