
![]()


From the Greek word Magnesia (a district of Thessaly)
|
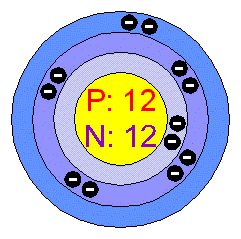
Atomic #- 12 Atomic Weight- 24.305 Electron Configuration - 1s22s22p63s2
Intensive Physical Properties - |
 |
||||||||||||||||||||
| Being an Alkaline Earth Metal, Magnesium forms a strong base when placed in water (An Alkaline solution) | |||||||||||||||||||||
|
Physical Properties Magnesium is a lightweight, silvery-white metal. Finely divided Magnesium ignites readily and burns with a hot white flame. Magnesium tarnishes slightly in air but is usually coated with a natural layer of MgO that protects it. |
 |
||||||||||||||||||||
|
Common Chemical Reactions There are a few compounds Magnesium forms readily: The surface layer of Mg reacts with Oxygen in the air to form an oxide that protects it from corrosion 2Mg(s) + O2(g)
3Mg(s) + N2(g)
Mg does not react with water to any significant extent but it does combine with steam in certain conditions Mg(s)
+ 2H2O(g)
Magnesium is very reactive with the halogens such as chlorine, Cl2 or bromine, Br2, and burns to form the dihalides magnesium(II) chloride, MgCl2, and magnesium(II) bromide, MgBr2, respectively. Mg(s)
+ Cl2(g)
Mg(s)
+ Br2(g)
|
|||||||||||||||||||||
|
A Few Isotopes
|
Magnesium's radioisotopes have very short
half lives; spanning from 1.3sec to 21 hours. Ex: 27Mg has a half life of 9.45 min |
||||||||||||||||||||
|
Sources of Magnesium Magnesium is never found as a free element in nature but it is the eighth most abundant element on earth. Magnesium can be found in minerals such as Magnesite and Dolomite (common parts of Granite) but it is most abundant in sea water. Magnesium salts can also be found in the earth where deposits of sea salts can be found in large quantities, including South Africa and Mt. Vesuvius, Italy. |
|||||||||||||||||||||
|
Uses of Magnesium
|
 |
||||||||||||||||||||
|
Magnesium in Biology Magnesium has several uses in Biology. 50 % of our Magnesium is in our bones while 50% is in intercellular fluid. While providing structure for bones Mg also has other uses. Most of these arise from deficiency however a Mg excess can result in kidney failure and diarrhea. Signs of excess magnesium can be similar to magnesium deficiency and include mental status changes, nausea, diarrhea, appetite loss, muscle weakness, difficulty breathing, extremely low blood pressure, and irregular heartbeat |
Excess= kidney failure / squirts Deficiency= nausea / muscle weakness |
||||||||||||||||||||
|
History of Magnesium In 1618 a farmer at Epsom in England attempted to give his cows water from a well. This they refused to drink because of the water's bitter taste. However the farmer noticed that the water seemed to heal scratches and rashes. The fame of Epsom salts spread. Eventually they were recognized to be magnesium sulphate, MgSO4. Black recognized magnesium as an element in 1755. It was isolated by Davy in 1808 who electrolyzed a mixture of magnesia (magnesium oxide, MgO) and mercuric oxide (HgO). Davy's first suggestion for a name was magnium but the name magnesium is now used.
|
Discovered by:
Joseph Black (1755) |
||||||||||||||||||||